neutrons in titanium|Titanium protons neutrons electrons : Clark How Many Protons, Neutrons and Electrons Does Titanium Have? By Farhan Sadik August 22, 2024August 22, 2024. Titanium is a classified transition metal . Watch Lara Diabla Arab porn videos for free, here on Pornhub.com. Discover the growing collection of high quality Most Relevant XXX movies and clips. No other sex tube is more popular and features more Lara Diabla Arab scenes than Pornhub! Browse through our impressive selection of porn videos in HD quality on any device you own.
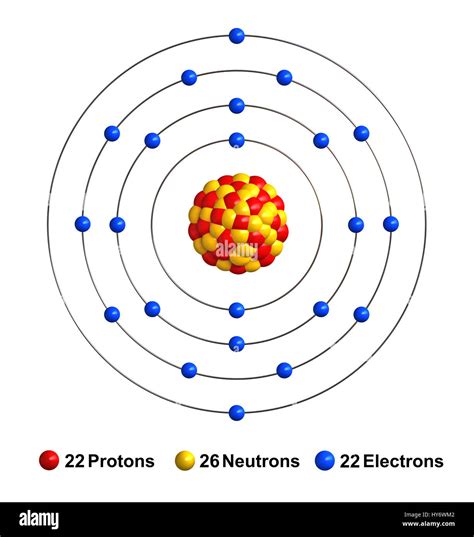
neutrons in titanium,Protons and Neutrons in Titanium. Titanium is a chemical element with atomic number 22 which means there are 22 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.

Atomic Number – Protons, Electrons and Neutrons in Titanium. Titanium is a .Titanium protons neutrons electrons The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the . How Many Protons, Neutrons and Electrons Does Titanium Have? By Farhan Sadik August 22, 2024August 22, 2024. Titanium is a classified transition metal .Titanium metal is used as an alloying agent with metals including aluminum, iron, molybdenum and manganese. Alloys of titanium are mainly used in aerospace, aircraft and engines where strong, lightweight, .
Name: Titanium Symbol: Ti Atomic Number: 22 Atomic Mass: 47.867 amu Melting Point: 1660.0 °C (1933.15 K, 3020.0 °F) Boiling Point: 3287.0 °C (3560.15 K, 5948.6 °F) .
Titanium is the 22nd element in the periodic table and has a symbol of Ti and atomic number of 22. It has an atomic weight of 47.867 and a mass number of 48. Titanium has . Titanium-50 is a stable isotope containing 28 neutrons. 5.18% of natural titanium is titanium-50. Bar of ultra-high purity titanium crystals. Credit: Heinrich Pnoik/Creative Commons
Titanium is a chemical element of the periodic table with chemical symbol Ti and atomic number 22 with an atomic weight of 47.8671 u and is classed as transition metal and is .neutrons in titaniumOverview. Titanium is found in the middle of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Titanium is a transition metal and is part of Group 4 (IVB). .Number of neutrons: 26 n 0: Number of electrons: 22 e-From Wikipedia, the free encyclopediaTitanium is a chemical element with symbol Ti and atomic number 22. It is a lustrous transition metal with a silver color, low density and high strength. . Titanium was discovered in 1791 by the clergyman and geologist William Gregor as an inclusion of .
Neutrons in most abundant isotope: 26: Electron shells: 2,8,10,2 : Electron configuration: [Ar] 3d 2 4s 2: Density @ 20 o C: 4.50 g/cm 3: . Titanium is not found freely in nature but is found in minerals such as rutile .
Naturally occurring titanium (22 Ti) is composed of five stable isotopes; 46 Ti, 47 Ti, 48 Ti, 49 Ti and 50 Ti with 48 Ti being the most abundant (73.8% natural abundance).Twenty-one radioisotopes have been characterized, with the most stable being 44 Ti with a half-life of 60 years, 45 Ti with a half-life of 184.8 minutes, 51 Ti with a half-life of 5.76 minutes, and 52 .Titanium is a transition metal and is part of Group 4 (IVB). Titanium was one of the first elements to be discovered by modern chemists. The "modern" chemistry period begins after the middle of the eighteenth century. . The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of .Name: Titanium Symbol: Ti Atomic Number: 22 Atomic Mass: 47.867 amu Melting Point: 1660.0 °C (1933.15 K, 3020.0 °F) Boiling Point: 3287.0 °C (3560.15 K, 5948.6 °F) Number of Protons/Electrons: 22 Number of Neutrons: 26 Classification: Transition Metal Crystal Structure: Hexagonal Density @ 293 K: 4.54 g/cm 3 Color: silverish Atomic Structure Thus, the number of electrons in Titanium is 22. Summary. Number of Protons in Titanium. The number of protons can be found by knowing the atomic number of that atom. Number of Protons in Titanium = Atomic number of Titanium = 22; Number of Neutrons in Titanium. The number of neutrons can be found by subtracting the atomic . Almost all of the mass of an atom is contained within a tiny (and therefore extremely dense) nucleus which carries a positive electric charge and almost all of the volume of an atom consists of empty space in which electrons reside (Figure \(\PageIndex{1}\)). The extremely small mass of the electron (1/1840 the mass of the . In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Titanium (Ti). From the Period. If Z=22 the element is titanium; everything follows from the atomic number. Z=22. What does this mean? It means that there are 22 massive, positively charged, fundamental particles in the element's nucleus. Given this, the element is unquestionably titanium. Given that there are 22 positive charges in the nucleus, the neutral element .

Hydrogen saturation of titanium-based materials exposed to irradiation with resonance neutrons with an energy of 0.1 MeV is considered. Radioactive scandium 22 Sc 46, gamma-quanta with energy of 889 and 1120 keV, and hydrogen form during nuclear reactions in titanium.The intensity of the gamma radiation depends on the .If you look lower in the sheet you will notice that titanium atom has 22 protons and 26 neutrons that will intuitively sum up to an atomic mass of 48. However, there are no less than 5 stable isotopes of titanium 46Ti, .For most elements other than hydrogen, isotopes are named for their mass number. For example, carbon atoms with the usual 6 neutrons have a mass number of 12 (6 protons + 6 neutrons = 12), so they are called carbon-12. Carbon atoms with 7 neutrons have an atomic mass of 13 (6 protons + 7 neutrons = 13). These atoms are the isotope called .Neutrons: 26 Shell structure: 2,8,10,2 Electron configuration: [Ar]3d24s2 Oxidation state: 4 Crystal structure: Hexagonal. Titanium has a very high strength-to-weight ratio and is corrosion-resistant. Most titanium is used in the form of titanium dioxide (TiO 2). Its silvery metallic color makes it very useful in paints, paper and plastics.Titanium-49 is a stable isotope of the chemical element titanium, which has 27 neutrons in the atomic nucleus in addition to the element-specific 22 protons, resulting in a mass number of 49. 49 Ti makes up 5.41% of the composition of Earth´s titanium.. There are no practical applications for the isotopically pure nuclide. See also: list of Titanium isotopes.
neutrons in titanium Titanium protons neutrons electrons The scientific paper presents studies of the radiation-protective features of a polymer composite material for protection against space radiation based on poly-tetra-fluoro-ethylene, Bi 2 O 3 bismuth oxide, WC tungsten carbide, B 4 C boron carbide, TiH 1.7 titanium hydride shot. The composite provides for a high degree of protection against . Specific KOWARI neutron diffraction strain scanner setup is shown in Fig. 3. Considering the weak scattering of titanium and localized texturing, experimental gauge volume of neutron beam was set as 2 × 2 × 2 mm 3 for point measurements of strains in longitudinal, transverse, and normal directions. Both bulk and stress-free samples used . The samples of niobium, zirconium and titanium were made into foils with the diameter about 18 mm and the thickness about 0.2 or 0.3 mm. The weight of each foil was determined with an analytic balance (± 0.01 mg).Neutron energy was determined by the cross section ratio method of 90 Zr(n,2n) 89 Zr to 93 Nb(n,2n) 92m Nb reactions (Lewis .
Titanium - Atomic Mass - Atomic Weight - Ti. The Titanium atomic mass is the mass of an atom. The density of a substance strongly depends on its atomic mass and also on the atomic number density. . (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section .
neutrons in titanium|Titanium protons neutrons electrons
PH0 · Titanium, Chemical Element
PH1 · Titanium protons neutrons electrons
PH2 · Titanium Protons Neutrons Electrons (A
PH3 · Titanium Facts
PH4 · Titanium Element Facts
PH5 · Titanium (Ti)
PH6 · Titanium
PH7 · Protons, Neutrons, Electrons for Titaniu
PH8 · How Many Protons, Neutrons and Electrons Does Titanium Have?
PH9 · Chemical Elements.com